
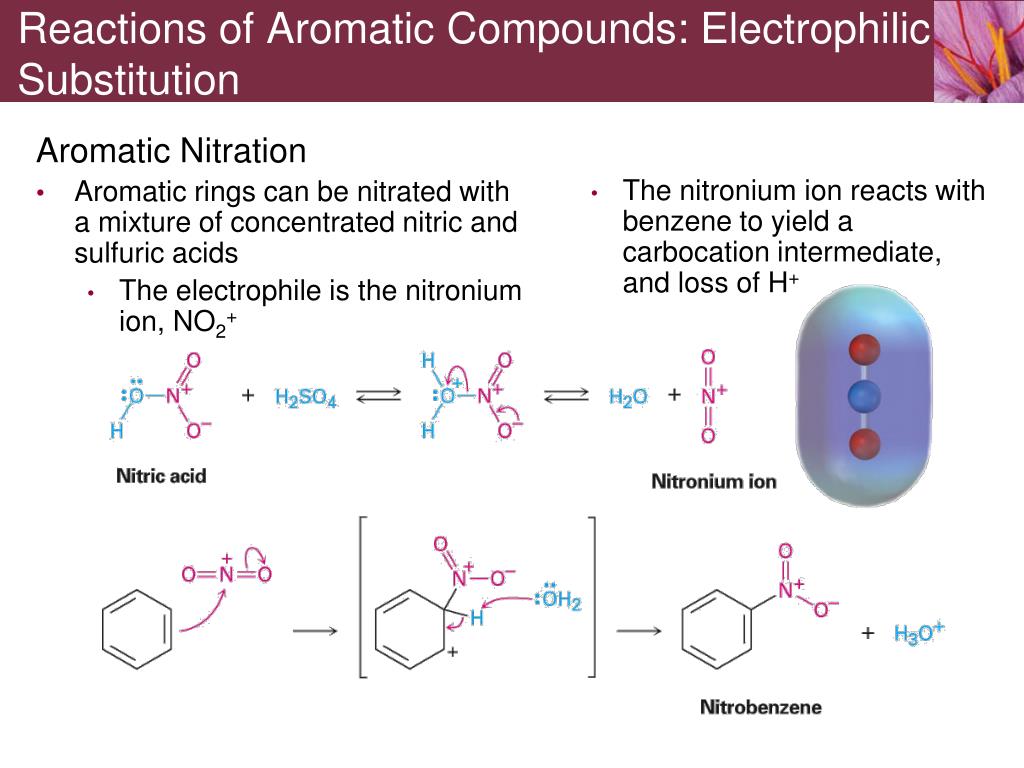
The catalysts and co-reagents serve to generate the strong electrophilic species needed to effect the initial step of the substitution. Arenes contain double bonds just like alkenes but they do not undergo electrophilic addition because these would result to their loss of ring aromaticity. Since the reagents and conditions employed in these reactions are electrophilic, these reactions are commonly referred to as Electrophilic Aromatic Substitution. Electrophilic aromatic substitution is a chemical reaction in which a hydrogen atom or a functional group from an aromatic compound is substituted with. Aromatic compounds or arenes undergo substitution reactions, in which the aromatic hydrogen is replaced with an electrophile, hence their reactions proceed via electrophilic substitution. Many substitution reactions of benzene have been observed, the five most useful are listed below (chlorination and bromination are the most common halogenation reactions). And it simplifies things to think about this as being the electrophile in your mechanism for electrophilic aromatic substitution, even though. For now, let’s see how the activation and ortho, para regioselectivity works by the inductive and resonance effects. There is now a need for a concise and general, but detailed and up-to-date, overview. Numerous studies have been carried out to provide an understanding of the nature of its reactivity pattern. \)Įxamples of Electophilic Aromatic Substitution (EAS) The following table summarizes the activating and deactivating groups and their directing effect in electrophilic aromatic substitution reactions: You can check this post for more details about the activating and deactivating groups. In the last few articles, we talked about the key electrophilic aromatic substitution reactions and the synthetic strategies based on the ortho, meta. Electrophilic aromatic substitution (EAS) is one of the most widely researched transforms in synthetic organic chemistry.


 0 kommentar(er)
0 kommentar(er)
